
Chemistry, 30.05.2020 06:00 caldonia2018
Balance the equation NH3+O2--->N2+H2O given 1.37 mol of the reactant NH3 , determine the corresponding amount of O2. Answer in units of mol.?
Find the corresponding amount of N2. Answer in units of mol.
Find the corresponding amount of H2O. Answer in units of mol.
Given 3.82 mol of the product N2, find the corresponding amount of NH3. Answer in units of mol.
Find the corresponding amount of O2. Answer in units of mol.
Find the corresponding amount of N2. Answer in units of mol.
Find the corresponding amount of H2O. Answer in units of mol.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 15:00
Theoretically, which metal should be the most reactive? hydrogen lithium francium fluorine
Answers: 1

Chemistry, 22.06.2019 04:00
How do scientists think that gravity affected the formation of our solar system?
Answers: 1

Chemistry, 22.06.2019 20:00
Which of the following would not diffuse through the plasma membrane by means of simple diffusion? 1 oxygen 2 glucose 3 a steroid hormone 4 a lipid soluble vitamin
Answers: 3

Chemistry, 22.06.2019 23:00
What is the energy in joules of a mole of photons associated with visible light of wavelength 486 nm?
Answers: 3
You know the right answer?
Balance the equation NH3+O2--->N2+H2O given 1.37 mol of the reactant NH3 , determine the correspo...
Questions

Physics, 17.04.2020 21:26


Social Studies, 17.04.2020 21:26

English, 17.04.2020 21:26



Biology, 17.04.2020 21:26

Mathematics, 17.04.2020 21:26

Mathematics, 17.04.2020 21:26

English, 17.04.2020 21:26


History, 17.04.2020 21:26

Biology, 17.04.2020 21:26

Mathematics, 17.04.2020 21:26

Chemistry, 17.04.2020 21:26





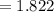 moles
moles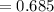 moles
moles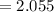 moles
moles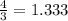 moles of Oxygems
moles of Oxygems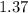 moles of NH3 shall combine with
moles of NH3 shall combine with 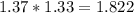 moles of oxygen (O2)
moles of oxygen (O2)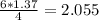 moles of water
moles of water

