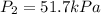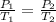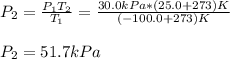Chemistry, 30.05.2020 19:02 hectorav6619
The temperature of a sample of gas in a steel
tank at 30.0 kPa is increased from -100.0°C to
25.0 °C. What is the final pressure inside the
tank?
A. 5.17 kPa
B. 51.7 kPa
C. 517 kPa
D. 5170 kPa

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:30
Which uses electromagnetic radiation to discover the properties and composition of bodies in space? space probe space station space shuttle space observatory
Answers: 2

Chemistry, 22.06.2019 02:30
When you perform this reaction, what could remain at the end of the reaction? check all that apply. excess reactant aqueous copper chloride excess reactant aluminum oxygen product solid copper carbon dioxide product aqueous aluminum chloride water
Answers: 2

Chemistry, 22.06.2019 13:00
Lab reagent, hypothesis test.a reference solution used as a lab reagent is purported to have a concentration of 5 mg/dl. six samples are taken from this solution and the following concentrations are recorded: (5.32, 4.88, 5.10, 4.73, 5.15, 4.75) mg/dl.these six measurements are assumed to be an srs of all possible measurements from solution.they are also assumed to have a standard deviation of 0.2, a normal distributin, and a mean concentration equal to the true concentration of the solution.carry out a significance test to determine whether these six measurements provide reliable evidence that the true concentration of the solution is actually not 5 mg/dl.
Answers: 1

Chemistry, 22.06.2019 13:30
Which is true of a liquid? it has a definite volume but not a definite mass.it has a definite mass but not a definite volume.it has a definite volume but not a definite shape.it has a definite shape but not a definite volume.
Answers: 2
You know the right answer?
The temperature of a sample of gas in a steel
tank at 30.0 kPa is increased from -100.0°...
tank at 30.0 kPa is increased from -100.0°...
Questions



Chemistry, 20.11.2020 23:30


Mathematics, 20.11.2020 23:30


English, 20.11.2020 23:30


Mathematics, 20.11.2020 23:30

English, 20.11.2020 23:30

Mathematics, 20.11.2020 23:30



Physics, 20.11.2020 23:30



Mathematics, 20.11.2020 23:30

Mathematics, 20.11.2020 23:30

Spanish, 20.11.2020 23:30