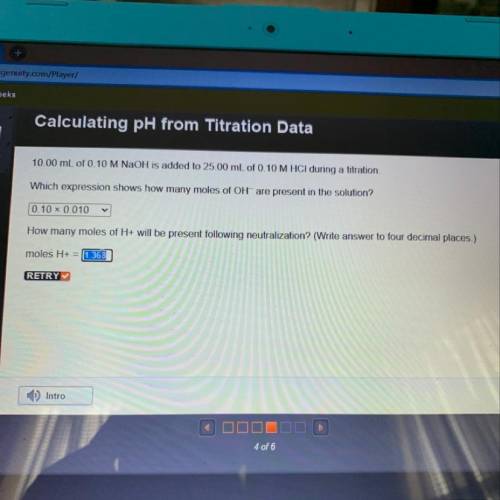
10.00 mL of 0.10 M NaOH is added to 25.00 mL of 0.10 M HCl during a titration,
Which expressio...

10.00 mL of 0.10 M NaOH is added to 25.00 mL of 0.10 M HCl during a titration,
Which expression shows how many moles of OH- are present in the solution?
How many moles of H+ will be present following neutralization? (Write answer to four decimal places.)
moles H+ =

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:30
How many moles of air are there in a human lung with a volume of 2.4 l at stp? explain your answer
Answers: 1


Chemistry, 22.06.2019 22:30
Consider a culture medium on which only gram-positive organisms such as staphylococcus aureus colonies can grow due to an elevated nacl level. a yellow halo surrounds the growth, indicating the bacterium fermented a sugar in the medium, decreasing the ph as a result and changing the color of a ph indicator chemical. this type of medium would be referred to as a differential and enrichment culture.
Answers: 2

Chemistry, 23.06.2019 09:30
If the solubility of a gas in water is 1.22g/2.75 atm, what is it’s solubility (in g/l) at 1.0 atm
Answers: 1
You know the right answer?
Questions



Biology, 18.11.2020 05:30

Social Studies, 18.11.2020 05:30


Mathematics, 18.11.2020 05:30

Spanish, 18.11.2020 05:30

Biology, 18.11.2020 05:30

Mathematics, 18.11.2020 05:30







Social Studies, 18.11.2020 05:30


Mathematics, 18.11.2020 05:30

Law, 18.11.2020 05:30

Mathematics, 18.11.2020 05:30



