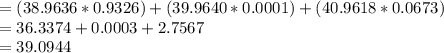Chemistry, 31.05.2020 03:02 SoccerHalo
Potassium has three naturally occurring isotopes. According to one source of data, Potassium-39 (mass 38.9637 amu) makes up 93.26%, potassium-40 (mass 39.9640 amu) makes up just 0.01%, and potassium-41 (mass 40.9618 amu) makes up 6.73%. What is the average atomic mass of potassium based on these relative abundances

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Which of the following is a compound? a.carbon b.oxygen c.hydrogen d.water
Answers: 2

Chemistry, 23.06.2019 01:00
If i had 2 m naoh solution, what does the 2 m stand for? 2 molar, but 2 of a solute in 1
Answers: 1


Chemistry, 23.06.2019 11:20
Try to reduce the amount of leftover ingredients by changing the amount of one, two, or all three starting ingredients. show your stoichiometric calculations below. water 946.36 g sugar 196.86 g lemon juice193.37 g
Answers: 2
You know the right answer?
Potassium has three naturally occurring isotopes. According to one source of data, Potassium-39 (mas...
Questions

Arts, 25.03.2021 09:10


Social Studies, 25.03.2021 09:10

Mathematics, 25.03.2021 09:10

Health, 25.03.2021 09:10







Geography, 25.03.2021 09:10

English, 25.03.2021 09:10


Mathematics, 25.03.2021 09:10

Mathematics, 25.03.2021 09:10

Physics, 25.03.2021 09:10



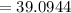
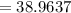 amu
amu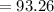 %
%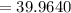 amu
amu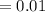 %
%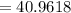 amu
amu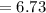 %
%