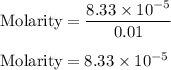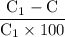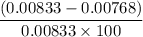You weighed out 0.020 g of your crude aspirin product in order to determine the amount of salicylic acid impurity. Following the procedure outlined in the manual, you dissolved the solid and diluted the solution to a final volume of 10.0 mL. If the absorbance of your sample solution was 1.07, what was the percent salicylic acid in your product

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:30
Acontrol during an experiment. might change remains constant does not exist does change
Answers: 1

Chemistry, 23.06.2019 02:00
Which of the following substances is the most soluble in water? a. sodium chloride b. methane c. bromine d. carbon
Answers: 1

Chemistry, 23.06.2019 11:30
How do you calculate the mass of a product when the amounts of more than one reactant are given?
Answers: 3

Chemistry, 23.06.2019 11:40
Which of the following would have the lowest average kinetic energy
Answers: 1
You know the right answer?
You weighed out 0.020 g of your crude aspirin product in order to determine the amount of salicylic...
Questions


Mathematics, 28.06.2019 15:00


Health, 28.06.2019 15:00

Mathematics, 28.06.2019 15:00

History, 28.06.2019 15:00

Mathematics, 28.06.2019 15:00




History, 28.06.2019 15:00


Physics, 28.06.2019 15:00

History, 28.06.2019 15:00





Mathematics, 28.06.2019 15:00

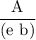
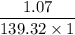 ( ε = 139.32)
( ε = 139.32)