Chemistry, 31.05.2020 06:58 ambercuevas2707
Attempt 1
The element carbon has two naturally occurring isotopes. The isotopic masses and abundances of these isotopes are shown
in the table.
Isotope
12c
13C
Isotopic mass (u)
12.00
Abundance (%)
98.93
13.00
1.07
Calculate the average atomic mass of carbon to two digits after the decimal point.
average atomic mass =

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 13:30
How many protons, electrons, and neutrons are in each of the following isotopes? a. zirconium-90 b. palladium-108 c. bromine-81 d. antimony-123
Answers: 1

Chemistry, 22.06.2019 19:10
Which statement correctly describes the phosphate ion, ? it is composed of one phosphorus atom and four oxygen atoms covalently bonded together, and there is a –3 charge distributed over the entire ion. it is composed of one phosphorus atom and four oxygen atoms covalently bonded together, and there is a –3 charge on the phosphorus atom. it is composed of one phosphorus atom and four oxygen atoms ionically bonded together, and there is a –3 charge distributed over the entire ion. it is composed of one phosphorus atom and four oxygen atoms ionically bonded together, and there is a –3 charge on the phosphorus atom.
Answers: 3

Chemistry, 22.06.2019 22:00
Imagine one batch of soup (batch “a”) is made with 8.19 g/can of salt, according to the recipe, and a second batch of soup (batch “b”) is made with 8.32 g/can of salt. explain which batch would be more resistant to frost damage if it is shipped a great distance in winter and explain why.
Answers: 2

Chemistry, 22.06.2019 22:30
Calculate the concentration of all species in a 0.165 m solution of h2co3.
Answers: 1
You know the right answer?
Attempt 1
The element carbon has two naturally occurring isotopes. The isotopic masses and abu...
The element carbon has two naturally occurring isotopes. The isotopic masses and abu...
Questions

Health, 28.09.2019 08:50


Geography, 28.09.2019 08:50

Biology, 28.09.2019 08:50



Chemistry, 28.09.2019 08:50

English, 28.09.2019 08:50

Mathematics, 28.09.2019 08:50

Geography, 28.09.2019 08:50

Mathematics, 28.09.2019 08:50


English, 28.09.2019 08:50


Mathematics, 28.09.2019 08:50


Mathematics, 28.09.2019 08:50


Mathematics, 28.09.2019 08:50

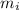 ) and the abundance of each isotope (
) and the abundance of each isotope (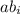 ) using the following expression.
) using the following expression.