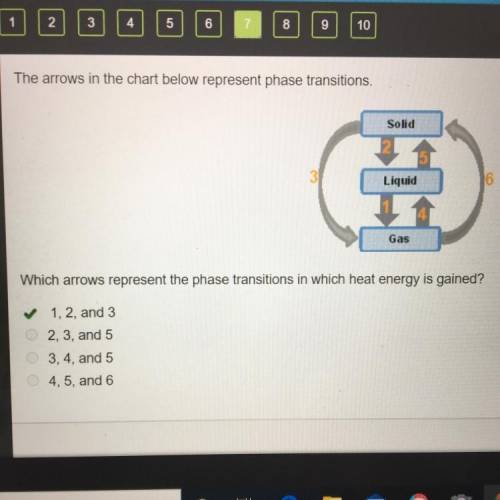
The arrows in the chart below represent phase transitions.
Which arrows represent the ph...

Chemistry, 02.06.2020 01:57 maystrenko53
The arrows in the chart below represent phase transitions.
Which arrows represent the phase transitions in which heat energy is gained?
1, 2, and 3
2, 3, and 5
3, 4, and 5
4, 5, and 6
A. Is the answer

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:30
Since the gas in your graduated cylinder is a mixture of butane and water vapor, you must determine the partial pressure of the butane, pbutane, alone. to do this, consult a reference and record the partial pressure of the water vapor, pwater, at the temperature you recorded. use the following formula to compute the partial pressure of the butane. pbutane = atmosphere - pwater use the following combined gas law formula and compute the volume that the butane sample will occupy at stp. (hint: convert both temperatures to kelvin.) pbutane x voriginal = pstandard x vfinal troom tstandard use the following ratio and proportion formula to determine the mass of butane needed to occupy a volume of 22.4 l at stp. grams of butane you used “x” grams of butane ml of butane corrected to stp = 22,400 ml compute the theoretical molar mass of butane based on its formula and the atomic masses on the periodic table. compare your experimental results from #3 to the theoretical value of #4, computing a percent error of your findings using this formula: % error = measured value - accepted value x 100 accepted value use the following ratio and proportion formula to determine the mass of butane needed to occupy a volume of 22.4 l at stp. need asap
Answers: 1

Chemistry, 22.06.2019 17:40
Which statement about hf is true? it is zero for any compound in its standard state. it is positive when the bonds of the product store more energy than those of the reactants. it is negative when a compound forms from elements in their standard states. it is zero for any element that is in the liquid state.
Answers: 1

Chemistry, 23.06.2019 01:30
Which is an example of a highly unstable isotope that is often used in fission reactions?
Answers: 1

Chemistry, 23.06.2019 06:10
How much would the freezing point of water decrease if 4 mol of nacl were added to 1 kg of water (kf=1.86 degrees c/(mol/kg) for water and i=2 for nacl a- 7.44 degrees c b- 14.88 c 3.72 d 1.86
Answers: 1
You know the right answer?
Questions

Biology, 03.12.2021 03:10





Social Studies, 03.12.2021 03:10

History, 03.12.2021 03:10

Mathematics, 03.12.2021 03:10

Mathematics, 03.12.2021 03:10

Computers and Technology, 03.12.2021 03:10

Mathematics, 03.12.2021 03:10

Mathematics, 03.12.2021 03:10

Geography, 03.12.2021 03:10

Computers and Technology, 03.12.2021 03:10




Mathematics, 03.12.2021 03:10

Mathematics, 03.12.2021 03:10

Business, 03.12.2021 03:10



