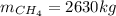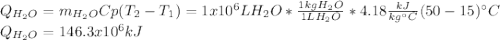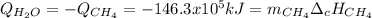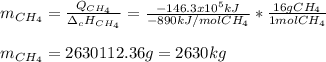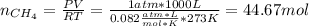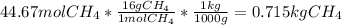En un balneario necesitan calentarse 1 millón de litros de agua anuales, subiendo la temperatura desde 15 ºC a 50 ºC y para ello utilizan calderas de gas natural (CH4), siendo la entalpía de combustión del metano de – 890 KJ/mol. Calcula:
a) El consumo anual de gas natural. Sol: 2630 Kg
b) El coste económico, anual, si el precio del m3, en condiciones normales, de gas natural es de 0,45 €. Datos Ce (H2O) = 4,18 KJ/Kg·K
Sol: 1657 €.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:30
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3

Chemistry, 22.06.2019 12:30
The melting point of sulfur is 115 °c and its boiling point is 445 °c. what state would sulfur be in at 200 °c?
Answers: 1

Chemistry, 22.06.2019 14:00
Displacement is the slope of a velocity vs. time graph a. true b. false
Answers: 1

Chemistry, 22.06.2019 14:50
Which of the following is most likely true about water in chemical systems? a) water dissolves nonpolar ionic compounds. b) water dissociates ionic compounds. c) water dissociates covalent molecules. d) water dissolves nonpolar covalent substances.
Answers: 1
You know the right answer?
En un balneario necesitan calentarse 1 millón de litros de agua anuales, subiendo la temperatura des...
Questions

Mathematics, 09.09.2020 22:01



English, 09.09.2020 22:01

Mathematics, 09.09.2020 22:01




Chemistry, 09.09.2020 22:01



Chemistry, 09.09.2020 22:01


English, 09.09.2020 22:01



Mathematics, 09.09.2020 22:01