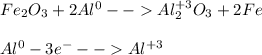Given the balanced equation representing a reaction:
Fe2O3 + 2Al Al2O3 + 2Fe
...

Chemistry, 01.06.2020 18:57 shayleewright
Given the balanced equation representing a reaction:
Fe2O3 + 2Al Al2O3 + 2Fe
During this reaction, the oxidation number of Al changes from
A) +3 to 0 as electrons are transferred
B) +3 to 0 as protons are transferred
C) 0 to +3 as electrons are transferred
D) 0 to +3 as protons are transferred

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:30
98 ! and brainliest plz ! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1

Chemistry, 22.06.2019 12:30
If anyone would be able to me out with these three questions it would be these are from the chem 2202 course.
Answers: 3

Chemistry, 22.06.2019 16:30
At 20°c, a sample of h2o liquid and a sample of co2 gas each have the same average kinetic energy. why is one a liquid and the other a gas at this temperature?
Answers: 1

Chemistry, 22.06.2019 18:00
What amount of heat is exchanged when 106.2 grams of substance y goes from a liquid at 35 degrees celsius to a solid at the same temperature? melting point of substance y = 35 degrees c; δhvaporization = 3.67 j/mol; δhfusion = 3.30 j/mol. mwsubstance y = 28.22 g/mol. −12.4 j −3.51 x 102 j 1.24 x 101 j 351 j
Answers: 1
You know the right answer?
Questions

Social Studies, 30.06.2019 18:30

Mathematics, 30.06.2019 18:30


Mathematics, 30.06.2019 18:30



Mathematics, 30.06.2019 18:30

Mathematics, 30.06.2019 18:30


Physics, 30.06.2019 18:30

Mathematics, 30.06.2019 18:30




Spanish, 30.06.2019 18:30





History, 30.06.2019 18:30