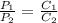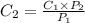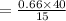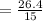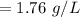Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Mrs. smith ordered a root beer float (vanilla ice cream + root beer). mrs. smith noticed that the three states of matter (solid, liquid, and gas) all existed simultaneously in her root beer float. a. identify each phase of matter in the root beer float. b. describe the particles of all three phases of matter in the root beer float. (how are the particles arranged and moving? ) c. identify one phase change you would see in a root beer float and described what causes this change.
Answers: 2

Chemistry, 22.06.2019 14:20
7. in the cycle, a virus integrates its dna into the host's dna, and its dna is replicated when the host dna is replicated. a. infectious b. retroviral c. lysogenic d.lytic
Answers: 1


Chemistry, 23.06.2019 04:00
Why must humans find substitutes for many minerals found on earth? (a) form at an extremely slow rate (b) controlled by other countries (c) too deep in the earth to collect
Answers: 1
You know the right answer?
The solubility of a gas in water is 0.66 g/L at 15 kPa of pressure. What is the solubility when the...
Questions


English, 02.09.2019 15:30

Social Studies, 02.09.2019 15:30


Geography, 02.09.2019 15:30

Social Studies, 02.09.2019 15:30

Social Studies, 02.09.2019 15:30


Mathematics, 02.09.2019 15:30



History, 02.09.2019 15:30


English, 02.09.2019 15:30


Mathematics, 02.09.2019 15:30



History, 02.09.2019 15:30

Social Studies, 02.09.2019 15:30