Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:30
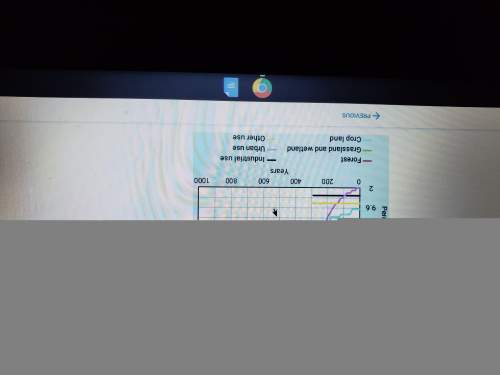
Jessica is traveling from miami, florida, to chicago, illinois. using the map, tell one way the land will change during the second half of her trip.
Answers: 1

Chemistry, 22.06.2019 13:30
Some animals that try to adapt to climate changes eventually die due to starvation, as climate change alters the web.
Answers: 2

Chemistry, 22.06.2019 16:10
Predict the reactants of this chemical reaction. that is, fill in the left side of the chemical equation. be sure the equation you submit is balanced. (you can edit both sides of the equation to balance it, if you need to.) note: you are writing the molecular, and not the net ionic equation. > cacl2(aq) + h20(l)
Answers: 2

Chemistry, 22.06.2019 17:00
Which property of a rock remains unchanged by mechanical weathering? a. total surface area b. size and shape c. mineral composition d. sharpness
Answers: 1
You know the right answer?
Vinegar (0.830M acetic acid) reacts with solid calcium hydroxide to produce carbon dioxide, water, a...
Questions

Spanish, 03.08.2019 06:10




Social Studies, 03.08.2019 06:10







Biology, 03.08.2019 06:10




Spanish, 03.08.2019 06:10

Spanish, 03.08.2019 06:10