
Chemistry, 02.06.2020 00:00 lgisselle629
Hydrogen, H2,and nitrogen, N2(g), combined to form ammonia, NH3(g):3H2+N2–>2NH3(g) what amount, in moles, of nitrogen will react 18 mols of hydrogen

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:00
Agas can holds 2.0 gal of gasoline. what is this quantity in cubic centimeters?
Answers: 2


Chemistry, 22.06.2019 13:50
Abeaker with 2.00×102 ml of an acetic acid buffer with a ph of 5.000 is sitting on a benchtop. the total molarity of acid and conjugate base in this buffer is 0.100 m. a student adds 4.70 ml of a 0.360 m hcl solution to the beaker. how much will the ph change? the pka of acetic acid is 4.740.
Answers: 1

Chemistry, 22.06.2019 17:30
The polymer used for the nonstick surface of cooking utensils is 24.0%c and 76%f by mass. what is the empirical formula of this polymer?
Answers: 2
You know the right answer?
Hydrogen, H2,and nitrogen, N2(g), combined to form ammonia, NH3(g):3H2+N2–>2NH3(g) what amount, i...
Questions

Biology, 23.07.2019 16:00

Mathematics, 23.07.2019 16:00

Chemistry, 23.07.2019 16:00







History, 23.07.2019 16:00

Mathematics, 23.07.2019 16:00


English, 23.07.2019 16:00



Biology, 23.07.2019 16:00

Mathematics, 23.07.2019 16:00

History, 23.07.2019 16:00


French, 23.07.2019 16:00

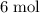 of
of 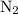 will react with
will react with 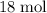 of
of 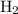 .
.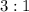 . That is:
. That is: 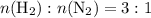 . That's the same as saying that for every one mole of
. That's the same as saying that for every one mole of 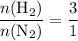 .
.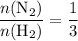 .
.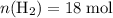 . The question is asking for
. The question is asking for 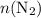 , the number of moles of
, the number of moles of 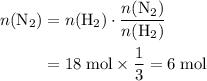 .
.

