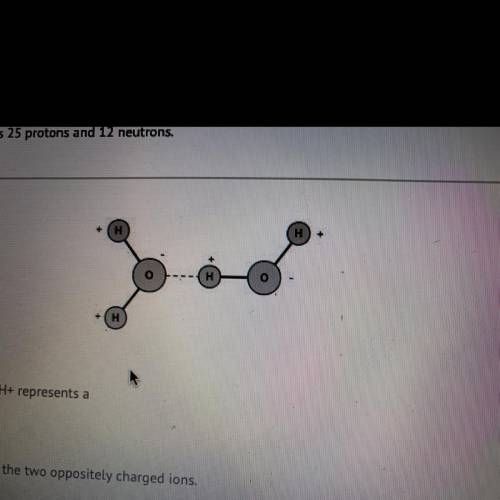
The dotted line between 0- and H+ represents a
A)
covalent bond between the two opposite...

The dotted line between 0- and H+ represents a
A)
covalent bond between the two oppositely charged ions.
B)
hydrogen bond, a weak bond between a hydrogen and an electronegative
atom.
C)
strong, short-distance bond that is responsible for the unique properties of
water.
polar covalent bond that results from the unequal and opposite charges of
the ions.
D)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Used the balanced equation 2h2+ o2 - -> 2h2o. if you have 7.2 grams of o2 , how many grams of h2o can you produce ?
Answers: 2

Chemistry, 22.06.2019 15:30
Which statement names the physical property of wood a. wood is softer than coal b. wood does not rust c. wood can rot d. wood can burn
Answers: 1

Chemistry, 22.06.2019 19:00
A4.86 g piece of metal was placed in a graduated cylinder containing 15.5 ml of water. the water level rose to 17.3 ml. what is the density of the metal. i need the steps of how to solve it to so i can use a formula to work out other problems.
Answers: 1

Chemistry, 23.06.2019 04:10
An unknown substance has been shown to have weak covalent bonds. which of the following is most likely a property of this substance? a. high ph b. high conductivity c. low melting point d. low flammability
Answers: 3
You know the right answer?
Questions

Mathematics, 30.03.2020 21:37

Engineering, 30.03.2020 21:37

English, 30.03.2020 21:37

Mathematics, 30.03.2020 21:37



Advanced Placement (AP), 30.03.2020 21:37


Mathematics, 30.03.2020 21:37

Mathematics, 30.03.2020 21:38


Mathematics, 30.03.2020 21:38


Mathematics, 30.03.2020 21:38

Social Studies, 30.03.2020 21:38

Mathematics, 30.03.2020 21:38

Mathematics, 30.03.2020 21:38

Biology, 30.03.2020 21:38

Mathematics, 30.03.2020 21:38



