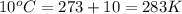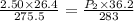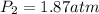Chemistry, 03.06.2020 07:57 Crtive6538
7. A 26.4-ml sample of ethylene gas, C2H4, has a pres-sure of 2.50 atm at 2.5°C. If the
volume is increased to 36.2 mL and the temperature is raised to 10°C, what is the
new pressure. (Hint: Three variables have been given so what equation will you
use?)

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 16:30
Energy is released during which phase changes? check all that apply. boiling condensing depositing freezing melting subliming
Answers: 2

Chemistry, 22.06.2019 09:20
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1

Chemistry, 22.06.2019 17:00
How can a give a full method for the experiment of separating sand from water by filtration? 1-materials 2-steps 3-conclusion also for water and salt separated by the evaporation or distillation process
Answers: 1

Chemistry, 22.06.2019 21:50
Liquid from a brewery fermentation contains 10% ethanol and 90% water. part of the fermentation product (50,000 kg/h) is pumped to a distillation column on the factory site. under current operating conditions, a distillate of 45% ethanol and 55% water is produced from the top of the column at a rate of one-tenth that of the feed. what is the composition of the waste "bottoms" from the still?
Answers: 2
You know the right answer?
7. A 26.4-ml sample of ethylene gas, C2H4, has a pres-sure of 2.50 atm at 2.5°C. If the
volume...
volume...
Questions




Business, 03.12.2020 18:30



Mathematics, 03.12.2020 18:30

English, 03.12.2020 18:30

Health, 03.12.2020 18:30

Physics, 03.12.2020 18:30

Mathematics, 03.12.2020 18:30


History, 03.12.2020 18:30



Mathematics, 03.12.2020 18:30

Spanish, 03.12.2020 18:30

Mathematics, 03.12.2020 18:30


Mathematics, 03.12.2020 18:30

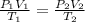
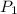 = initial pressure of gas = 2.50 atm
= initial pressure of gas = 2.50 atm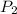 = final pressure of gas = ?
= final pressure of gas = ?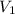 = initial volume of gas = 26.4 ml
= initial volume of gas = 26.4 ml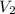 = final volume of gas = 36.2 ml
= final volume of gas = 36.2 ml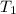 = initial temperature of gas =
= initial temperature of gas = 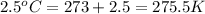
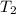 = final temperature of gas =
= final temperature of gas =