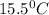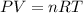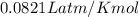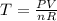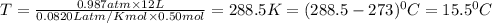Chemistry, 03.06.2020 09:57 muravyevaarina
Calculate the temperature of a 0.50 mol sample of a gas at 0.987 atm and a volume of 12 L.
-7 C
11 C
15.5 C
288 C

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:00
What is the molecular formula for a compound that is 46.16% carbon, 5.16% hydrogen, and 48.68% fluorine? the molar mass of the compound is 156.12 g/mol
Answers: 2

Chemistry, 22.06.2019 13:00
The molality of calcium chloride (cacl2) in an aqueous solution is 2.46 m. what is mole fraction of the solute?
Answers: 3

Chemistry, 22.06.2019 19:10
How does the atmosphere to make earth livable? check all that apply. causes the seasons contains oxygen provides warmth creates important nutrients blocks harmful energy from the sun plz like !
Answers: 2

Chemistry, 23.06.2019 04:20
The graph shows one consequence of urban sprawl. how did urban sprawl contribute to the change in biodiversity
Answers: 2
You know the right answer?
Calculate the temperature of a 0.50 mol sample of a gas at 0.987 atm and a volume of 12 L.
Questions

Mathematics, 05.11.2020 14:00


Mathematics, 05.11.2020 14:00

Mathematics, 05.11.2020 14:00

English, 05.11.2020 14:00

Mathematics, 05.11.2020 14:00


Mathematics, 05.11.2020 14:00

English, 05.11.2020 14:00


English, 05.11.2020 14:00

Health, 05.11.2020 14:00

Biology, 05.11.2020 14:00



Mathematics, 05.11.2020 14:00

Social Studies, 05.11.2020 14:00


SAT, 05.11.2020 14:00