Chemistry, 04.06.2020 13:12 werdtotheblue691
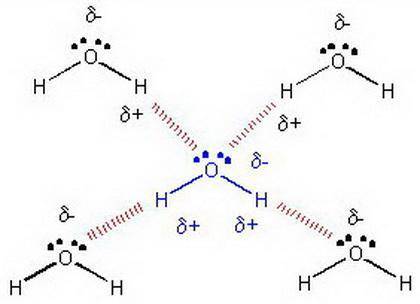
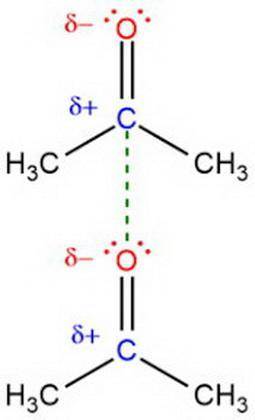
Relacione el punto de ebullición del agua y la acetona (propanona), con la facilidad de a evaporación de estas sustancias (volatilidad), considerando las fuerzas intermoleculares.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 05:30
Match the following vocabulary terms to their definitions. 1. amount of energy required to change 1 gram of material from the solid to the liquid state at its melting point 2. a measure of the kinetic energy of the particles of a substance 3. the amount of heat energy required to raise the temperature of 1 gram of liquid water from 14.5°c to 15.5°c 4. amount of energy required to change 1 gram of material from the liquid to the gaseous state at its boiling point 5. the amount of energy required to change 1 gram of a substance 1°c a. temperature b. latent heat of vaporization c. latent heat of fusion d. calorie e. specific heat
Answers: 1

Chemistry, 22.06.2019 05:30
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 1

You know the right answer?
Relacione el punto de ebullición del agua y la acetona (propanona), con la facilidad de a evaporació...
Questions

Computers and Technology, 04.03.2021 23:20

Mathematics, 04.03.2021 23:20

Chemistry, 04.03.2021 23:20




Mathematics, 04.03.2021 23:20

Mathematics, 04.03.2021 23:20





History, 04.03.2021 23:20




Social Studies, 04.03.2021 23:20


Mathematics, 04.03.2021 23:20

Business, 04.03.2021 23:20