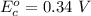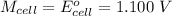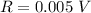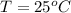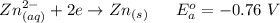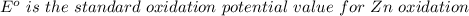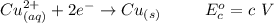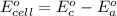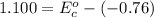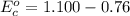Chemistry, 04.06.2020 13:20 saggin2454
Using the cell voltage measured for the first cell studied, with cell chemistry Zn/Zn^2+ \ Cu^2+/Cu, and the known half life potential for Zn^2/Zn calculate the reduction potential for Cu^2+/Cu and enter value below.
The information received for this problem were the values obtained during an online lab:
Cu xM cell voltage:1.100 V
Range: 0.005 V
Temp: 25 degrees C

Answers: 3


Another question on Chemistry

Chemistry, 23.06.2019 00:30
How many moles of co2 are produced during the complete combustion of 3.6 moles of c2h6
Answers: 1

Chemistry, 23.06.2019 01:00
Chromium(iii) sulfate is a transition metal compound containing the metal chromium and the polyatomic ion sulfate. the oxidation state of chromium in this compound is , and the chemical formula of the compound is ( ) . reset next
Answers: 3

Chemistry, 23.06.2019 02:00
Which of these is a density dependent factor? a. epidemic b. earthquake c. drought d. hurricane
Answers: 2

Chemistry, 23.06.2019 05:50
Which of the following isotopes has the same number of neutrons as phosphorus-31?
Answers: 1
You know the right answer?
Using the cell voltage measured for the first cell studied, with cell chemistry Zn/Zn^2+ \ Cu^2+/Cu,...
Questions

Health, 18.11.2019 15:31

Computers and Technology, 18.11.2019 15:31

Geography, 18.11.2019 15:31


French, 18.11.2019 15:31


Mathematics, 18.11.2019 15:31

Physics, 18.11.2019 15:31





English, 18.11.2019 15:31


Biology, 18.11.2019 15:31



Geography, 18.11.2019 15:31

Mathematics, 18.11.2019 15:31

Health, 18.11.2019 15:31

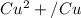 is
is