
Chemistry, 04.06.2020 19:01 babbity2009
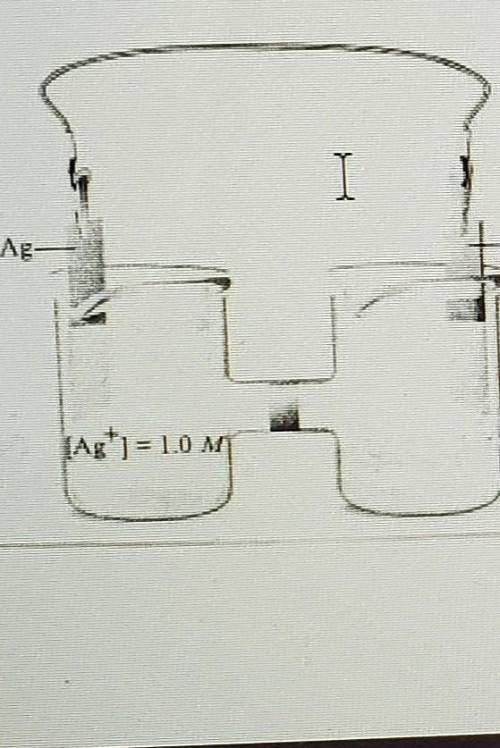
1. Consider the concentration cell below: Identify the anode, cathode, and the direction of electron flow. Calculate the cell potential at 25°C. The (Ag*) in the right-hand beaker is 1.0x10-8M

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 16:30
In which direction will the following reaction go if the standard reduction potentials are 0.80 v for ag/ag+ and –0.44 v for fe/fe2+? ag+ + fe → ag + fe2+ a.)forward b.)the reaction cannot occur. c.) not enough information is given. d.) reverse
Answers: 1

Chemistry, 22.06.2019 07:20
After watching the video "zinc strip in copper nitrate solution", and reading the instructions, click on the link labeled "start" just below the drawing of the pencil tip. follow the direction to complete the 3x3 grid. answer the below questions for the portion of the activity in which sn(s) is placed in agno3(aq)
Answers: 1

Chemistry, 22.06.2019 09:30
Apump contains 0.5 l of air at 203 kpa.you draw back on the piston of the pump, expanding the volume until the pressure reads 50.8 kpa. what is the new volume of the air pump
Answers: 2

Chemistry, 22.06.2019 10:30
What woukd most likely be the transmittance at a 0.70 m solution of solute a? a) 7.6%b) 1.1%c)4.0%d)4.6%
Answers: 1
You know the right answer?
1. Consider the concentration cell below: Identify the anode, cathode, and the direction of
electro...
Questions

History, 28.04.2021 19:50





Mathematics, 28.04.2021 19:50


Mathematics, 28.04.2021 19:50


Mathematics, 28.04.2021 19:50



English, 28.04.2021 19:50

French, 28.04.2021 19:50



Mathematics, 28.04.2021 19:50





