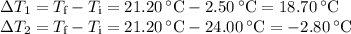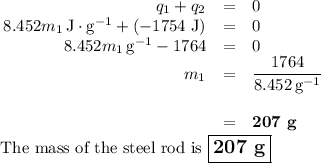CHEM EXPERT NEEDED***
A volume of 150.0 mL of H2O is initially at 24.00 °C. A chilled steel rod at 2.50 °C is placed in the water and the final temperature of the system is 21.20 °C.
Specific heat of water = 4.184 J/(g⋅∘C) and the specific heat of steel = 0.452 J/(g⋅∘C)
Write the equation and calculate the mass of the rod.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:00
As you watch a surfer ride a wave towards the shoreline, what is the shoreline? a) displacement reference b) reference point c) coordinate plane d) cartesian boundary
Answers: 1

Chemistry, 22.06.2019 11:00
The diagram below shows the different phase transitions that occur in matter. which arrow represents the transition in which dew is formed?
Answers: 1

Chemistry, 23.06.2019 06:10
2. what two items do autotrophs take from the environment to produce their food? 3. what are the two items that are released during transpiration from leaves? 4. what are the two membranes of the system? a.what are the two stages of photosynthesis? what are the two parts of photosynthesis?
Answers: 2

You know the right answer?
CHEM EXPERT NEEDED***
A volume of 150.0 mL of H2O is initially at 24.00 °C. A chilled steel rod at...
Questions

Mathematics, 12.08.2020 06:01

Health, 12.08.2020 06:01




History, 12.08.2020 06:01