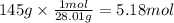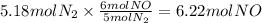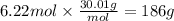Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 16:00
The chemical equation below shows the reaction of sodium (na) and chlorine (cl) to form sodium chloride (nacl). 2na + cl2 → 2nacl in this equation, which of the following is a reactant? i. sodium ii. chlorine iii. sodium chloride
Answers: 1

Chemistry, 22.06.2019 19:00
A4.86 g piece of metal was placed in a graduated cylinder containing 15.5 ml of water. the water level rose to 17.3 ml. what is the density of the metal. i need the steps of how to solve it to so i can use a formula to work out other problems.
Answers: 1

Chemistry, 22.06.2019 20:00
If one fission reaction of a uranium-235 atom produced two neutrons, how many neutrons would be released if the chain reaction occurred three more times?
Answers: 1
You know the right answer?
How many grams of NO are required to produce 145 g of N2 in the following reaction?
4NH3(g) + 6NO(g...
Questions



Mathematics, 11.11.2020 18:10


History, 11.11.2020 18:10

Mathematics, 11.11.2020 18:10

Mathematics, 11.11.2020 18:10


Social Studies, 11.11.2020 18:10


Chemistry, 11.11.2020 18:10

Computers and Technology, 11.11.2020 18:10





Mathematics, 11.11.2020 18:10

Chemistry, 11.11.2020 18:10


English, 11.11.2020 18:10