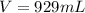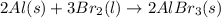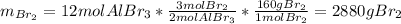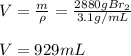Chemistry, 05.06.2020 08:58 codycollier
Consider the following unbalanced equation. How many Liters of bromine are needed to produce 12 moles of Aluminum bromide? The density of bromine is 3.1 g/mL. Al (s) + Br2 (l)= AlBr3 (s)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:20
In a reaction equation, where are the products located? a.) above the arrow b.) to the right of the arrow c.) to the left of the arrow d.) below the arrow
Answers: 2

Chemistry, 23.06.2019 07:00
Achemist who studies water samples did a demonstration of how to test for lead in water. she added a clear solution of potassium iodide to a clear solution of lead nitrate. then a yellow swirling solid formed in the liquid. what is most likely true about the yellow solid?
Answers: 3

Chemistry, 23.06.2019 08:00
The biosphere of the earth is made up of . a. inorganic b. organic
Answers: 2

Chemistry, 23.06.2019 13:30
The process by which liquid water changes into water vapour is called what ?
Answers: 2
You know the right answer?
Consider the following unbalanced equation. How many Liters of bromine are needed to produce 12 mole...
Questions


Mathematics, 25.09.2020 14:01


Physics, 25.09.2020 14:01




History, 25.09.2020 14:01


Mathematics, 25.09.2020 14:01



Mathematics, 25.09.2020 14:01

Biology, 25.09.2020 14:01

English, 25.09.2020 14:01

Mathematics, 25.09.2020 14:01

English, 25.09.2020 14:01



English, 25.09.2020 14:01