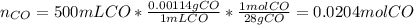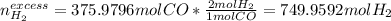Chemistry, 05.06.2020 16:00 nxusasmangaliso1191
Methanol CH3OH is the simplest of the alcohols . It is sinthesized by the reaction of hydrogen and carbon monoxide.
CO + H2 > CH3OH
a) If 500 ml CO and 750 mol H2 are present, which is the limiting reactant?
b) How many mols of excess reactant remain unchange?
c) How many moles of CH3OH are formed ?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:10
Think about how you can use le chatelier’s principle to find possible solutions to the design problem. describe at least two ways to increase the yield (amount) of ammonia based on this principle.
Answers: 2

Chemistry, 22.06.2019 07:30
Compare and contrast the bohr model and the electron cloud models of the atom.
Answers: 1

Chemistry, 22.06.2019 18:30
Which of the following nuclei would be the least stable a 2 protons, 2 neutrons b 1 proton 1 neutron c 1 proton 3 neutrons d 1 proton 2 neutrons
Answers: 3

Chemistry, 22.06.2019 23:00
What element has similar physical and chemical properties as boron.
Answers: 1
You know the right answer?
Methanol CH3OH is the simplest of the alcohols . It is sinthesized by the reaction of hydrogen and c...
Questions


English, 25.03.2021 19:40





Mathematics, 25.03.2021 19:40

Mathematics, 25.03.2021 19:40


Mathematics, 25.03.2021 19:40



Chemistry, 25.03.2021 19:40

Chemistry, 25.03.2021 19:40


English, 25.03.2021 19:40

English, 25.03.2021 19:40

Mathematics, 25.03.2021 19:40

History, 25.03.2021 19:40