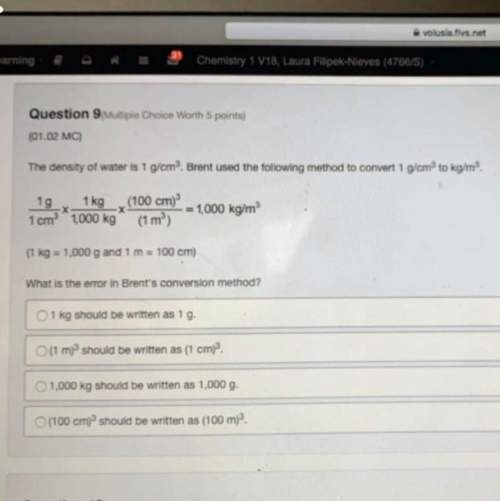
Consider the reaction.
2HF(g) +
H2(g) + F2(g)
At equilibrium at 600 K, the...

Chemistry, 05.06.2020 23:02 chhimmidemg
Consider the reaction.
2HF(g) +
H2(g) + F2(g)
At equilibrium at 600 K, the concentrations are as follows.
[HF] = 5.82 x 10-2 M
[H2] = 8.4 x 10-3M
[F2] = 8.4 x 10-3M
What is the value of Kea for the reaction expressed in scientific notation?
O 2.1 x 10-2
102.1 x 102
1.2 x 103
0 1.2 x 10-3

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Based on the equation and the information in the table, what is the enthalpy of the reaction?
Answers: 2

Chemistry, 22.06.2019 07:10
Which of these conditions most likely produces an unstable isotope?
Answers: 2

Chemistry, 22.06.2019 16:00
Which factor is likely to impact the possible number of compounds ?
Answers: 1

Chemistry, 22.06.2019 20:30
Identify the correct mole ratio for each substance. sodium chloride (nacl) na: cl = 1: ammonium nitrate (nhno) h: o = 4:
Answers: 1
You know the right answer?
Questions

Mathematics, 21.08.2019 13:30

Biology, 21.08.2019 13:30

Mathematics, 21.08.2019 13:30


Mathematics, 21.08.2019 13:30

Mathematics, 21.08.2019 13:30




Social Studies, 21.08.2019 13:30

Health, 21.08.2019 13:30

Social Studies, 21.08.2019 13:30

Biology, 21.08.2019 13:30


Biology, 21.08.2019 13:30



Mathematics, 21.08.2019 13:30

History, 21.08.2019 13:30