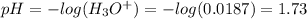A 1-liter solution contains 0.494 M hydrofluoric acid and 0.371 M potassium fluoride. Addition of 0.408 moles of hydrochloric acid will: (Assume that the volume does not change upon the addition of hydrochloric acid.)
a. Raise the pH slightly
b. Lower the pH slightly
c. Raise the pH by several units
d. Lower the pH by several units
e. Not change the pH
f. Exceed the buffer capacity

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 12:00
Ican determine the molar mass of an element by looking on the under the atomic mass for the element. for example the molar mass of phosphorus is 30.974 grams/mole. avogadro’s number tells me the amount of representative particles in 1 mole of any substance. this means 12.011 gram sample of carbon and a 32.0 gram sample of sulfur have the same number of atoms.
Answers: 1

Chemistry, 22.06.2019 14:30
Is a pencil falling to the floor anon contact force, a force, or a contact force
Answers: 1

Chemistry, 22.06.2019 21:30
How many liters of 3.0 m naoh solution will react with 0.60 liters of 4.0 m h2so4? h2so4 + naoh → na2so4 + h2o 1.2 l 1.6 l 2.4 l 2.8 l
Answers: 3

Chemistry, 23.06.2019 19:30
The total amount of fresh water on earth is estimated to be 3.73 x 10^8 km^3. what is this volume in cubic meters? in cubic feet?
Answers: 1
You know the right answer?
A 1-liter solution contains 0.494 M hydrofluoric acid and 0.371 M potassium fluoride. Addition of 0....
Questions

Mathematics, 04.03.2021 21:00

Mathematics, 04.03.2021 21:00

Biology, 04.03.2021 21:00



Mathematics, 04.03.2021 21:00

English, 04.03.2021 21:00

English, 04.03.2021 21:00

History, 04.03.2021 21:00

Mathematics, 04.03.2021 21:00

Mathematics, 04.03.2021 21:00


Mathematics, 04.03.2021 21:00


Mathematics, 04.03.2021 21:00

Mathematics, 04.03.2021 21:00



Mathematics, 04.03.2021 21:00

![pH = pKa + log(\frac{[KF]}{[HF]})](/tpl/images/0677/3728/a79c6.png)
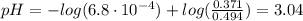
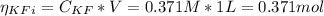
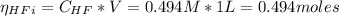
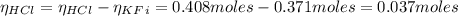
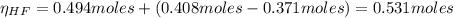
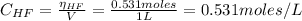
![Ka = \frac{[H_{3}O^{+}][F^{-}]}{[HF]}](/tpl/images/0677/3728/2de73.png)
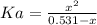 (2)
(2)