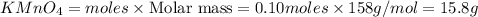Chemistry, 06.06.2020 05:01 kestegag7162
2KMnO4= K2MnO4+ MnO2+O2 how many grams of KMnO4 are required to produce 1.60 grams of O2

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 17:30
Observation and experimentation have led many scientists to accept a theory about the origin of the universe. this theory is called the big bang theory. scientific evidence collected and observed by scientists around the world suggests that the universe is ever expanding from a hot and dense initial state. what makes this a scientific theory? (2 points)
Answers: 2

Chemistry, 22.06.2019 19:00
Convert the temperature of dry ice, –77 ∞c, into degrees fahrenheit and kelvin.
Answers: 2

Chemistry, 22.06.2019 20:30
Consider the following unbalanced equation for the combustion of hexane: αc6h14(g)+βo2(g)→γco2(g)+δh2o(g) part a balance the equation. give your answer as an ordered set of numbers α, β, γ, use the least possible integers for the coefficients. α α , β, γ, δ = nothing request answer part b determine how many moles of o2 are required to react completely with 5.6 moles c6h14. express your answer using two significant figures. n n = nothing mol request answer provide feedback
Answers: 2

Chemistry, 23.06.2019 01:30
Ascientist conducted an experiment and discovered that certain plants grow faster when given a particular amount of fertilizer. anouther scientist conducted the same experiment and got similar results. which concept does this best illustrate? a) repetition b) replication c) precision d) validity
Answers: 2
You know the right answer?
2KMnO4= K2MnO4+ MnO2+O2 how many grams of KMnO4 are required to produce 1.60 grams of O2...
Questions


Biology, 03.06.2020 22:57

Mathematics, 03.06.2020 22:57

Biology, 03.06.2020 22:57

Chemistry, 03.06.2020 22:57


History, 03.06.2020 22:57



Physics, 03.06.2020 22:57

Mathematics, 03.06.2020 22:57

Mathematics, 03.06.2020 22:57

Mathematics, 03.06.2020 22:57

Mathematics, 03.06.2020 22:57






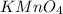 will be required to produce 1.60 grams of
will be required to produce 1.60 grams of 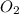
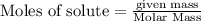
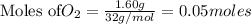
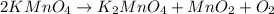
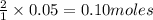 of
of