Answers: 1


Another question on Chemistry

Chemistry, 23.06.2019 07:00
An unknown substance is a white solid at room temperature and has a melting point of 78 °c. which of the following substances is most likely to be the identity of the unknown sample? a. naphthalene, a molecular solid with the formula c10h8 b. silica, a network solid held together by covalent bonds with the formula sio2 c. calcium chloride, an ionic compound with the formula cacl2 d. water, an molecular compound with the formula h2o
Answers: 2

Chemistry, 23.06.2019 08:40
The activation energy for this reaction is 75 kj·mol–1. the enzyme catalase (found in blood) lowers the activation energy to 8.0 kj·mol–1. at what temperature would the non-catalyzed reaction need to be run to have a rate equal to that of the enzyme-catalyzed reaction at 25°c?
Answers: 2


Chemistry, 23.06.2019 21:30
Enzymes are always consumed by catalyzing a reaction increase the rate at which a reaction occurs sometimes increase the amount of energy necessary to initiate a reaction catalyze reactions that release energy, but not those that consume energy
Answers: 1
You know the right answer?
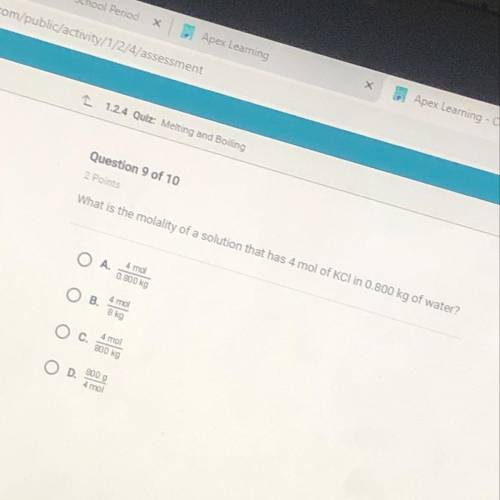
What is the molality of a solution that has 4 mol of KCI in 0.800 kg of water
...
...
Questions

Mathematics, 30.11.2020 23:30

Mathematics, 30.11.2020 23:30

English, 30.11.2020 23:30

Mathematics, 30.11.2020 23:30

History, 30.11.2020 23:30

Mathematics, 30.11.2020 23:30


History, 30.11.2020 23:30



History, 30.11.2020 23:30

Biology, 30.11.2020 23:30



History, 30.11.2020 23:30

Arts, 30.11.2020 23:30

English, 30.11.2020 23:30



History, 30.11.2020 23:30