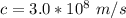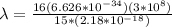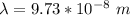Chemistry, 06.06.2020 04:57 jasonskatesallday
A sample tube consisted of atomic hydrogen in their ground state. A student illuminated the atoms with monochromatic light, that is, light of a single wavelength. If only two separate emission lines in the visible region are observed, what is the wavelength (or wavelengths) of the incident radiation?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:30
14. complete and balance the equations for the single displacement reactions. a. zn + pb(no3)2 -> b. al + niso4 -> 15. complete and balance the equations for the double displacement reactions. a. agno3(aq) + nacl(aq) -> b. mg(no3)2(aq) + koh(aq) -> 16. complete and balance the equations for the combustion reactions. a. __ ch4 + o2 -> b. __ c3h6 + o2 -> c. + o2 ->
Answers: 2

Chemistry, 22.06.2019 12:10
Building glycogen from glucose molecules is an example of
Answers: 3

Chemistry, 22.06.2019 13:40
Can someone me with 6 to 10 plz this is for masteries test.
Answers: 1

Chemistry, 23.06.2019 08:30
Benzonitrile (c6h5cn) is reduced to two different products depending on the reducing agent used. treatment with lithium aluminum hydride followed by water forms k, which has a molecular ion in its mass spectrum at 107 and the following ir absorptions: 3373, 3290, 3062, 2920, and 1600 cm-1. treatment with a milder reducing agent forms l, which has a molecular ion in its mass spectrum at 106 and the following ir absorptions: 3086, 2850, 2820, 2736, 1703, and 1600 cm-1. l shows fragments in its mass spectrum at m/z = 105 and 77. propose structures for k and l and choose an explanation for how this could be concluded.
Answers: 3
You know the right answer?
A sample tube consisted of atomic hydrogen in their ground state. A student illuminated the atoms wi...
Questions



Mathematics, 05.05.2020 20:23







Medicine, 05.05.2020 20:23

Physics, 05.05.2020 20:23


World Languages, 05.05.2020 20:23





Mathematics, 05.05.2020 20:24


Mathematics, 05.05.2020 20:24

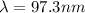



![\Delta E = R_H [\frac{1}{n_i^2} -\frac{1}{n_f^2} ]](/tpl/images/0678/3328/e4fbe.png)
![\Delta E= R_H [\frac{1}{1^2} -\frac{1}{4^2} ]](/tpl/images/0678/3328/c5793.png)


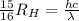

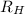 is the Rydberg constant with a value of
is the Rydberg constant with a value of