CaCO3(s)

Chemistry, 06.06.2020 08:58 CjTheStudentLOL
17. Consider the reaction shown and identify the statement that is not true.
825°C
CaCO3(s)
+ CaO(s) + CO2(g)
a.
This reaction is balanced as written.
b. The reactant must be heated for this reaction to occur.
c. The products are a solid and a gas.
d. Water must be present for this reaction to occur.
There are no solutions used in this reaction.
e.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:30
Plz mark brainliest 30 points 1) find the momentum of a 12 kg snowball that is rolling with a velocity of 9 m/s. 2) an 8 ball with a mass of .5 kg is sitting at rest. it is hit by the cue ball (1 kg) traveling at 2.5 m/s. if the cue ball is at rest after the collision, how fast is the 8 ball traveling after the collision? 3) two football players are running toward each other. if the offensive player is 75 kg and is running 8 m/s, how fast must the 60 kg defensive player run in order for the two players to hit and stop?
Answers: 1


Chemistry, 22.06.2019 19:30
How might this scientific phenomena be explained? a paper clip floats on water.
Answers: 1

Chemistry, 23.06.2019 01:00
Which of the following is in the lanthanide family? a) uranium b) promethium c) silver d) gold
Answers: 2
You know the right answer?
17. Consider the reaction shown and identify the statement that is not true.
825°C
CaCO3(s)
CaCO3(s)
Questions


Mathematics, 23.08.2021 19:00


Computers and Technology, 23.08.2021 19:00

Mathematics, 23.08.2021 19:00

History, 23.08.2021 19:00


Mathematics, 23.08.2021 19:00


Social Studies, 23.08.2021 19:00

Business, 23.08.2021 19:00


Mathematics, 23.08.2021 19:00


Mathematics, 23.08.2021 19:00



Computers and Technology, 23.08.2021 19:00


Mathematics, 23.08.2021 19:00

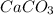 decomposition is:
decomposition is: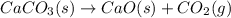
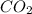 as product.
as product.

