Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Which of the following ocean acidification? are the most likely side effects of a ph less than 7.0 in the ocean b. more metal salts altering the ocean chemistry c. dissolution of the shells of marine organisms d. both a & b e. all of the above.
Answers: 3


Chemistry, 22.06.2019 10:50
8) a mixture of he, ne and ar has a pressure of 7.85 atm. if the ne has a mole fraction of 0.47 and 8) ar has a mole fraction of 0.23, what is the pressure of he? a) 4.2 atm b) 3.7 atm c) 5.5 atm d) 2.4 atm e) 1.8 atm
Answers: 1

Chemistry, 22.06.2019 12:00
A5.000 g sample of niso4 h2o decomposed to give 2.755 g of anhydrous niso4. what is the formula of the hydrate? what is the full chemical name for the hydrate? what is the molar mass of the hydrate? niso4•_h2o what is the mass % of water in the hydrate?
Answers: 1
You know the right answer?
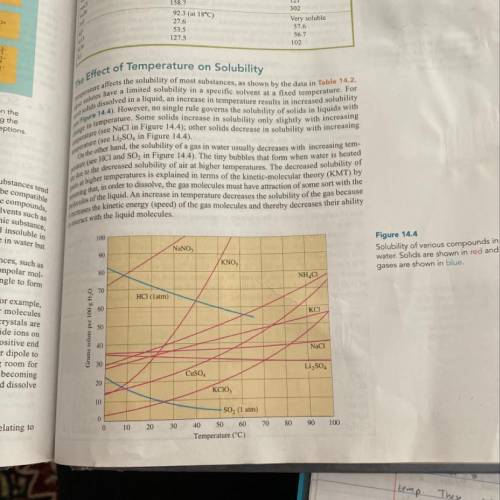
9. If 40. g Li2SO4 are added to 75.0 g water at 40°C, will the solution be saturated
or unsaturated...
Questions



Mathematics, 13.01.2020 06:31


Mathematics, 13.01.2020 06:31

Spanish, 13.01.2020 06:31





Mathematics, 13.01.2020 06:31

Chemistry, 13.01.2020 06:31



Biology, 13.01.2020 06:31

English, 13.01.2020 06:31

Mathematics, 13.01.2020 06:31

Mathematics, 13.01.2020 06:31


Mathematics, 13.01.2020 06:31