
Chemistry, 07.06.2020 00:01 irishvball7
A manganese electrode was oxidized electrically. If the mass of the electrode decreased by 225 mg during the passage of 1580 coulombs, what was the oxidation state of the manganese produced

Answers: 1


Another question on Chemistry



Chemistry, 22.06.2019 18:10
Given the following at 25c calculate delta hf for hcn (g) at 25c. 2nh3 (g) +3o2 (g) + 2ch4 (g) > 2hcn (g) + 6h2o (g) delta h rxn= -870.8 kj. delta hf=-80.3 kj/mol for nh3 (g), -74.6 kj/mol for ch4, and -241.8 kj/mol for h2o (g)
Answers: 1

Chemistry, 22.06.2019 20:30
The activation energy for the reaction no2(g)+co2(g)⟶no(g)+co(g) is ea = 300 kj/mol and the change in enthalpy for the reaction is δh = -100 kj/mol . what is the activation energy for the reverse reaction?
Answers: 3
You know the right answer?
A manganese electrode was oxidized electrically. If the mass of the electrode decreased by 225 mg du...
Questions

Mathematics, 11.02.2020 18:25

Mathematics, 11.02.2020 18:25



Medicine, 11.02.2020 18:25

Computers and Technology, 11.02.2020 18:25



Biology, 11.02.2020 18:25


Computers and Technology, 11.02.2020 18:25









Computers and Technology, 11.02.2020 18:25

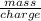
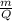 =
= 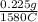 = 0.0001424 g/C
= 0.0001424 g/C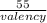 = 0.0001424 × 96,485
= 0.0001424 × 96,485

