
Chemistry, 07.06.2020 00:03 dylalove4963
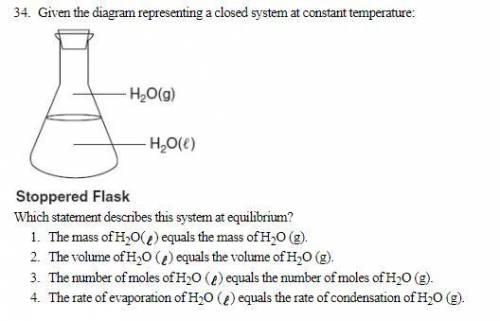
Given the diagram representing a closed system at constant temperature: Which statement describes this system at equilibrium? 1. The mass of H 2 O( ) equals the mass of H 2 O (g). 2. The volume of H 2 O ( ) equals the volume of H 2 O (g). 3. The number of moles of H2 O ( ) equals the number of moles of H2 O (g). 4. The rate of evaporation of H2 O ( ) equals the rate of condensation of H2 O (g).

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:00
In the analysis of hair and fiber samples, which does a compound comparison microscope allow for that a conventional compound microscope does not? a. simultaneous observation b. polarization c. fluorescence d. higher magnification
Answers: 2


Chemistry, 23.06.2019 10:30
Using the periodic table, complete the following. element: hydrogen symbol: h₂ molecular weight: g mass of one mole: g/mol
Answers: 2

Chemistry, 23.06.2019 18:00
If cos x =sin(20 + x) and 0 < x < 90, the value of x is > 3 , 35 , 350answer is 35, free 50 points
Answers: 1
You know the right answer?
Given the diagram representing a closed system at constant temperature: Which statement describes th...
Questions

History, 05.06.2020 01:03


English, 05.06.2020 01:03

Mathematics, 05.06.2020 01:03

Mathematics, 05.06.2020 01:03

Mathematics, 05.06.2020 01:03



Mathematics, 05.06.2020 01:03





Mathematics, 05.06.2020 01:03






English, 05.06.2020 01:03



