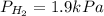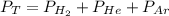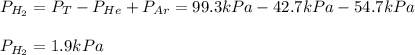Chemistry, 07.06.2020 01:00 montgomerykarloxc24x
The total pressure of a mixture of H2, He, and Ar is 99.3 kPa. The partial pressure of the He is 42.7 kPa, and the partial pressure of Ar is 54.7 kPa. What is the partial pressure of hydrogen

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Elements that do not have full outer electron shells will donate, share, or take electrons from other atoms. choose the items that have the correct binary ionic formula.
Answers: 2

Chemistry, 22.06.2019 17:50
Cryolite, na3alf6(s), an ore used in the production of aluminum, can be synthesized using aluminum oxide. start this question by first balance the chemical equation.1.) balance the equation: - alo3(s)+naoh(l)+hf(> na3alf6+h2o(g). 2.) if 17.5 kilograms of al2o3(s), 51.4 kilograms of naoh(l), and 51.4 kilograms of hf(g) react completely, how many kilograms of cryolite will be produced? 3.)which reactants will be in excess, (al2o3, naoh, or hf) 4.)what is the total mass of the excess reactants left over after the reaction is complete in kg?
Answers: 2

Chemistry, 22.06.2019 20:30
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 3

Chemistry, 23.06.2019 02:30
Which words or phrases identify layers of groundwater? check all that apply. water table kettle lake saturation zone underground lake sinkhole will give brainiest, answer quickly.
Answers: 1
You know the right answer?
The total pressure of a mixture of H2, He, and Ar is 99.3 kPa. The partial pressure of the He is 42....
Questions

English, 19.05.2021 01:40


Mathematics, 19.05.2021 01:40


Mathematics, 19.05.2021 01:40



Mathematics, 19.05.2021 01:40




Mathematics, 19.05.2021 01:40




Physics, 19.05.2021 01:40



Mathematics, 19.05.2021 01:40