
Chemistry, 07.06.2020 04:57 robert7248
Calculate the standard enthalpy of formation of NOCl(g) at 25 ºC, knowing that the standard enthalpy of formation of NO(g) at that temperature is 90.25 kJ / mol, and that the standard molar enthalpy of the reaction 2 NOCl(g) → 2 NO(g) + Cl2(g) is 75.5 kJ / mol at the same temperature.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Of the groups of elements below, which are most likely to gain electrons to become anions? a. alkali metal b. boron group c. halogen d. transition metal
Answers: 2

Chemistry, 22.06.2019 14:10
Precision can be defined as the o exact center of a data set. o reproducibility of a measured value. o correlation between two variables that are measured in a data set agreement between a measured value and an accepted value.
Answers: 2

Chemistry, 22.06.2019 19:30
Describe the forces both attractive and repulsive that occur as two atoms move closer together.
Answers: 1

Chemistry, 22.06.2019 20:30
Citric acid has a ph between 1 and 3. it is considered to be aa. weak acidb. weak basec. strong based. strong acid
Answers: 2
You know the right answer?
Calculate the standard enthalpy of formation of NOCl(g) at 25 ºC, knowing that the standard enthalpy...
Questions


English, 05.10.2019 18:00




Chemistry, 05.10.2019 18:00


Biology, 05.10.2019 18:00





History, 05.10.2019 18:00

Mathematics, 05.10.2019 18:00

Arts, 05.10.2019 18:00

Mathematics, 05.10.2019 18:00

Chemistry, 05.10.2019 18:00



History, 05.10.2019 18:00

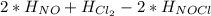
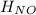 = 90.25 kJ/mol
= 90.25 kJ/mol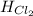 = 0 (For the formation of one mole of a pure element the heat of formation is 0, in this caseyou have as a pure compound the chlorine Cl₂)
= 0 (For the formation of one mole of a pure element the heat of formation is 0, in this caseyou have as a pure compound the chlorine Cl₂)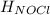 =?
=?

