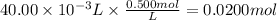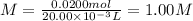Hydrobromic acid solution of unknown concentration is titrated with a 0.500M LiOH solution.
20.00mL of the acid are poured into an Erlenmeyer flask.
40.00mL of the base solution is required to reach the equivalence point.
What is the molarity of the Hydrobromic acid solution?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:10
What shape would a molecule with two bound groups and two lone pairs have?
Answers: 1

Chemistry, 22.06.2019 12:30
What is the percent composition of ca(oh)2? 37.7% ca, 53.0% o, and 10.3% h 45.5% ca, 38.2% o, and 16.3% h 54.0% ca, 43.0% o, and 2.7% h 64.7% ca, 27.0% o, and 8.3% h
Answers: 2

Chemistry, 22.06.2019 14:50
Which of the following is most likely true about water in chemical solutions?
Answers: 1

Chemistry, 22.06.2019 15:00
Many ionic compounds and a few highly polar covalent compounds are because they completely ionize in water to create a solution filled with charged ions that can conduct an electric current.
Answers: 1
You know the right answer?
Hydrobromic acid solution of unknown concentration is titrated with a 0.500M LiOH solution.
20.00mL...
Questions


Chemistry, 02.09.2020 04:01


Mathematics, 02.09.2020 04:01

Mathematics, 02.09.2020 04:01




Mathematics, 02.09.2020 04:01






Mathematics, 02.09.2020 04:01


Biology, 02.09.2020 04:01