
Chemistry, 09.06.2020 19:57 josephicarusmarrujo
At high temperatures one mole of hydrogen gas reacts with one mole of bromine gas to form hydrogen bromide. At a given temperature the equilibrium constant is 57.6. If at the same temperature, a mixture of 4.67 × 10^-3M bromine gas, 2.14 × 10^−3 hydrogen gas, and 2.40 × 10^−2M hydrogen bromide gas is made, then:
a. the system is at equilibrium.
b. the system is far from equilibrium and will shift to form more hydrogen gas.
c. the system is far from equilibrium and will shift to form more hydrogen bromide gas.
d. nothing can be deduced since we do not know whether the reaction is endothermic or exothermic.
e. nothing can be deduced since we do not know whether the equilibrium constant is Kc or Kp.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:30
This is a characteristic of the elements in the periodic table that shows a pattern. it may increase or decrease across or down the table.
Answers: 1

Chemistry, 22.06.2019 05:30
Compare and contrast physical changes with chemical changes.
Answers: 1

Chemistry, 22.06.2019 14:30
The three types is stress that act on earths rocks are compression, tension, and
Answers: 1

Chemistry, 22.06.2019 15:00
Why does a plastic bottle that is sealed at a high altitude change it’s shape when taken to lower altitude
Answers: 2
You know the right answer?
At high temperatures one mole of hydrogen gas reacts with one mole of bromine gas to form hydrogen b...
Questions

Mathematics, 20.10.2020 18:01



Computers and Technology, 20.10.2020 18:01




Geography, 20.10.2020 18:01

Mathematics, 20.10.2020 18:01



Chemistry, 20.10.2020 18:01



Mathematics, 20.10.2020 18:01


Computers and Technology, 20.10.2020 18:01



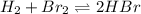
![Keq=\frac{[HBr]^2}{[H_2][Br_2]} =57.6](/tpl/images/0680/9707/6b896.png)
![Q=\frac{[HBr]^2}{[H_2][Br_2]} =\frac{(2.40x10^{-2})^2}{(4.67x10^{-3})(2.14x10^{-3})} \\\\Q=57.6](/tpl/images/0680/9707/53e8b.png)


