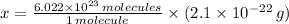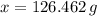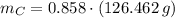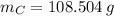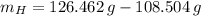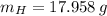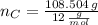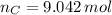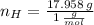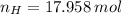Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Asample of aluminum foil contains 8.60 × 1023 atoms. what is the mass of the foil?
Answers: 1

Chemistry, 22.06.2019 11:00
An object becomes electrically charged when: electrons are created in it electrons from it are destroyed electrons are transferred to it protons from it are destroyed protons are created in it
Answers: 1

Chemistry, 22.06.2019 16:50
Assuming complete dissociation of the solute, how many grams of kno3 must be added to 275 ml of water to produce a solution that freezes at -14.5 c? the freezing point for pure water is 0.0 c and k_f is equal to 1.86 c/m
Answers: 3

Chemistry, 22.06.2019 19:30
Use the periodic table to find the molar mass of each element. molar mass h = g/mol molar mass s = g/mol molar mass o = g/mol
Answers: 3
You know the right answer?
Un hidrocarburo tiene como composición en masa: C= 85.8% ; H= 14.2% . Como dato nos brindan que una...
Questions

Computers and Technology, 29.08.2020 06:01







Mathematics, 29.08.2020 06:01






Physics, 29.08.2020 06:01




Mathematics, 29.08.2020 06:01


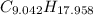
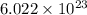 moléculas. La masa de un mol se determina mediante regla de tres simple:
moléculas. La masa de un mol se determina mediante regla de tres simple: