The disinfectant hydrogen peroxide (H2O2)
decomposes to form water and oxygen gas.
How much O...

Chemistry, 08.06.2020 22:57 mamas4539p79bw7
The disinfectant hydrogen peroxide (H2O2)
decomposes to form water and oxygen gas.
How much O2 will result from the decomposition of 2.22 mol of hydrogen peroxide?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:50
Consider the reaction: n2(g) + o2(g) ? 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 05:30
What is the mass of each element in a 324.8 sample of co2
Answers: 1

Chemistry, 22.06.2019 07:30
In a reaction (at equilibrium) that makes more moles of gas than it consumes, what is the effect of increasing the pressure?
Answers: 1

Chemistry, 22.06.2019 10:30
Find the number of grams of hcl needed to react completely with .50 moles of magnesium.
Answers: 1
You know the right answer?
Questions

Mathematics, 12.04.2021 02:00

Mathematics, 12.04.2021 02:00


Biology, 12.04.2021 02:10


History, 12.04.2021 02:10

Chemistry, 12.04.2021 02:10

Biology, 12.04.2021 02:10

Advanced Placement (AP), 12.04.2021 02:10

Mathematics, 12.04.2021 02:10

Mathematics, 12.04.2021 02:10



Computers and Technology, 12.04.2021 02:10


Health, 12.04.2021 02:10


Mathematics, 12.04.2021 02:10

Mathematics, 12.04.2021 02:10

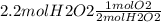 = 1.1 mol of O2
= 1.1 mol of O2

