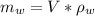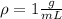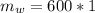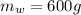Chemistry, 09.06.2020 23:57 jkw1222p0ttvq
A chemistry student is given 600. mL of a clear aqueous solution at 37° C. He is told an unknown amount of a certain compound X is dissolved in the solution. The student allows the solution to cool to 21° C. At that point, the student sees that a precipitate has formed. He pours off the remaining liquid solution, throws away the precipitate, and evaporates the water from the remaining liquid solution under vacuum. More precipitate forms. The student washes, dries and weighs the additional precipitate. It weighs 0.084 kg. Using only the information from above, can you calculate the solubility of X at 21° C?If yes, calculate it. Be sure your answer has a unit symbol and the right number of significant figures.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:00
Match the name of the following compound: mgso4 · h2omagnesium sulfate monohydratemagnesium (ii) sulfate monohydratemagnesium (ii) sulfate hydratemagnesium sulfate hydrate
Answers: 1


Chemistry, 22.06.2019 23:00
In which region is the substance in both the solid phase and the liquid phase? 1 2. 3 4 mark this and return save and exit next
Answers: 2

Chemistry, 23.06.2019 05:30
Awhite powder is added to a solution. the images show observations made before the powder is added, just after the powder has been added, and a little while later. (the liquid in the small beaker is phenol red solution.) what evidence shows that a chemical change has taken place?
Answers: 1
You know the right answer?
A chemistry student is given 600. mL of a clear aqueous solution at 37° C. He is told an unknown amo...
Questions


Mathematics, 29.04.2021 21:20

Mathematics, 29.04.2021 21:20




Social Studies, 29.04.2021 21:20


English, 29.04.2021 21:20


Physics, 29.04.2021 21:20


English, 29.04.2021 21:20







Mathematics, 29.04.2021 21:20

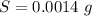
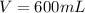
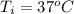
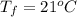
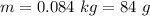
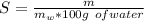
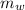 ) in the solution is mathematically represented as
) in the solution is mathematically represented as