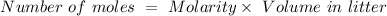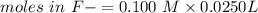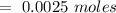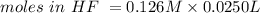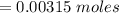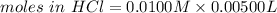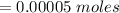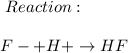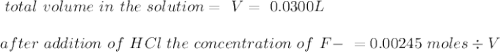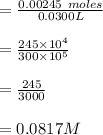Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 15:30
The reactions of photosynthesis occur in the of plant cell? a.mitochondria. b. lysosomes. c. chloroplasts. d. chlorophyll
Answers: 1

Chemistry, 22.06.2019 17:30
Aroller coaster is traveling at 13 mi./s when you purchase a hill that is 400 m long and down the hill exonerate at 4.0 m/s squared what is the final velocity of the posterior found your answer to the nearest number
Answers: 1

Chemistry, 22.06.2019 19:00
Which is the solubility product expression for caf2(s)?  [ca2+]/[f–]2  [ca2+][f2–]  [ca]+[f]2  [ca2+][f–]2
Answers: 3

Chemistry, 22.06.2019 21:00
Once similarity and one difference between a mixture of elements and a mixture of compounds
Answers: 3
You know the right answer?
Consider a solution containing 0.100 M fluoride ions and 0.126 M hydrogen fluoride. The concentratio...
Questions

Chemistry, 20.12.2020 14:00

English, 20.12.2020 14:00



History, 20.12.2020 14:00


Mathematics, 20.12.2020 14:00

Chemistry, 20.12.2020 14:00


Mathematics, 20.12.2020 14:00


English, 20.12.2020 14:00


Chemistry, 20.12.2020 14:10


Geography, 20.12.2020 14:10

Mathematics, 20.12.2020 14:10

Mathematics, 20.12.2020 14:10


Mathematics, 20.12.2020 14:10