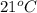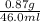Chemistry, 09.06.2020 14:57 brennae8529
A geochemist in the field takes a 46.0 mL sample of water from a rock pool lined with crystals of a certain mineral compound X. He notes the temperature of the pool, 21°C, and caps the sample carefully. Back in the lab, the geochemist filters the sample and then evaporates all the water under vacuum. Crystals of X are left behind. The researcher washes, dries and weighs the crystals. They weigh 0.87 g.
Required:
Using only the information above, can you calculate the solubility of X in water at 21°C? If yes, calculate it.

Answers: 2


Another question on Chemistry

Chemistry, 23.06.2019 02:00
Calculate the molarity of each aqueous solution: a. 78.0 ml of 0.240 m naoh diluted to 0.250 l with water b. 38.5 ml of 1.2 m hno3 diluted to 0.130 l with water
Answers: 1

Chemistry, 23.06.2019 04:00
If you are told to get 100 ml of stock solution to use to prepare smaller size sample for an experiment, which piece of glassware would you use?
Answers: 3

Chemistry, 23.06.2019 06:30
1.17 mol hcl and 2.5 mol naoh react according to the equation hcl + naoh -> nacl + h2o . if the limiting reactant is hcl, determine the amount of excess reactant that remains. answer in units of mol.
Answers: 1

Chemistry, 23.06.2019 11:30
Which of the following is a property of an acid solution? a. slippery to the touch b. ph less than 7 c. turns red litmus paper blue d. bitter taste
Answers: 1
You know the right answer?
A geochemist in the field takes a 46.0 mL sample of water from a rock pool lined with crystals of a...
Questions

Social Studies, 24.03.2021 17:10

Mathematics, 24.03.2021 17:10

Biology, 24.03.2021 17:10

English, 24.03.2021 17:10

Mathematics, 24.03.2021 17:10

English, 24.03.2021 17:10


Mathematics, 24.03.2021 17:10

Biology, 24.03.2021 17:10


Mathematics, 24.03.2021 17:10

Mathematics, 24.03.2021 17:10




Mathematics, 24.03.2021 17:10



Biology, 24.03.2021 17:10

Mathematics, 24.03.2021 17:10

 g/ml.
g/ml.