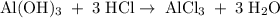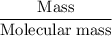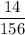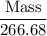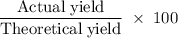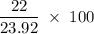Aluminum hydroxide is often present in antacids to neutralize stomach acid (HCl). If 14.0 g aluminum hydroxide is present in an antacid tablet, determine the theoretical yield of aluminum chloride produced when the tablet reacts with stomach acid. If the actual yield of the aluminum chloride from this tablet is 22.0 g, what is the percent yield?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:30
The first element on the periodic table of elements is carbon. a. true b. false
Answers: 2


Chemistry, 22.06.2019 09:00
Given the following reaction: c3h8+5o2=3co2+4h20 how many grams of co2 will be produced 7 g of c3h8 and 98 g of o2
Answers: 1

You know the right answer?
Aluminum hydroxide is often present in antacids to neutralize stomach acid (HCl). If 14.0 g aluminum...
Questions


Chemistry, 13.03.2021 01:00

Mathematics, 13.03.2021 01:00




Chemistry, 13.03.2021 01:00

Mathematics, 13.03.2021 01:00

Physics, 13.03.2021 01:00

Mathematics, 13.03.2021 01:00



Chemistry, 13.03.2021 01:00

Mathematics, 13.03.2021 01:00

Mathematics, 13.03.2021 01:00