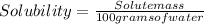A chemistry student is given of a clear aqueous solution at . He is told an unknown amount of a certain compound is dissolved in the solution. The student allows the solution to cool to . At that point, the student sees that a precipitate has formed. He pours off the remaining liquid solution, throws away the precipitates, and evaporates the water from the remaining liquid solution under vacuum. More precipitate forms. The student washes, dries and weighs the additional precipitate. It weighs 50,0 g. Using only the information above, can you calculate the solubility of X in water at 16. C. If you said yes, calculate it.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:40
Which type of precipitation would most likely form when the surface air temperature is slightly below freezing and the air temperature increases as you move upward away from the ground?
Answers: 2

Chemistry, 22.06.2019 14:00
Calculate the frequency of a wave in a spring toy. the wave has a speed of 1.1 meters per second and a wavelength of 0.1 meters. *
Answers: 2

Chemistry, 22.06.2019 16:00
Uranium can supply energy for the worlds electricity without admitting harmful greenhouse gases which of these statements best describes an outcome of uranium mining
Answers: 1

Chemistry, 23.06.2019 00:00
This statement about matter and its behavior is best classified as a
Answers: 1
You know the right answer?
A chemistry student is given of a clear aqueous solution at . He is told an unknown amount of a cert...
Questions




Computers and Technology, 06.04.2021 21:30

Mathematics, 06.04.2021 21:30

Mathematics, 06.04.2021 21:30

Mathematics, 06.04.2021 21:30

English, 06.04.2021 21:30


Mathematics, 06.04.2021 21:30

Social Studies, 06.04.2021 21:30

Mathematics, 06.04.2021 21:30








Physics, 06.04.2021 21:40