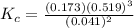Chemistry, 10.06.2020 18:57 ninaaforever
Ammonia will decompose into nitrogen and hydrogen at high temperature. An industrial chemist studying this reaction fills a tank with of ammonia gas, and when the mixture has come to equilibrium measures the amount of nitrogen gas to be 13. mol. Calculate the concentration equilibrium constant for the decomposition of ammonia at the final temperature of the mixture.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:30
Which answer lists the fundamental forces in order from strongest to weakest
Answers: 1

Chemistry, 22.06.2019 05:50
Significant figures are digits read directly from the measuring instrument plus one more digit, which is __ by the observer.
Answers: 2

Chemistry, 22.06.2019 10:30
Aglow stick contains a glass vial with chemicals. when the glow stick is bent, the vial breaks and the chemicals react to produce a glow. a science student observes that a glow stick kept in the freezer glows for a longer duration than a glow stick kept at room temperature. what conclusion can be drawn based on the observation? be sure to note the outcome and test variables in the conclusion.
Answers: 1

You know the right answer?
Ammonia will decompose into nitrogen and hydrogen at high temperature. An industrial chemist studyin...
Questions

English, 25.09.2019 23:00




English, 25.09.2019 23:00


Physics, 25.09.2019 23:00



Health, 25.09.2019 23:00


Chemistry, 25.09.2019 23:00

History, 25.09.2019 23:00

Mathematics, 25.09.2019 23:00

Health, 25.09.2019 23:00

Health, 25.09.2019 23:00




Mathematics, 25.09.2019 23:00

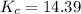
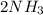 ↔
↔ 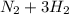
![[NH_3] = \frac{n_1}{V_1} = \frac{29}{75}](/tpl/images/0681/9995/b151c.png)
![[NH_3] = 0.387 \ M](/tpl/images/0681/9995/5ee5d.png)
![[N_2] = \frac{n_2}{V_2}](/tpl/images/0681/9995/a9b63.png)
![[N_2] = 0.173 \ M](/tpl/images/0681/9995/f89ee.png)
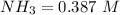

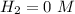
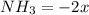 (this implies that it losses two moles of concentration )
(this implies that it losses two moles of concentration )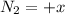 (this implies that it gains 1 moles)
(this implies that it gains 1 moles) (this implies that it gains 3 moles)
(this implies that it gains 3 moles)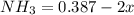
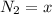
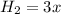
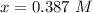
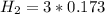

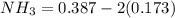
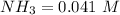
![K_c = \frac{[N_2][H_2]^3}{[NH_3]^2}](/tpl/images/0681/9995/10463.png)