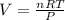Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:50
Consider the equilibrium system: 2icl(s) ⇄ i2(s) + cl2(g) which of the following changes will increase the total amount of of cl2 that can be produced? all of the listed answers are correct decreasing the volume of the container removing the cl2 as it is formed adding more icl(s) removing some of the i2(s)
Answers: 1

Chemistry, 22.06.2019 06:30
If 1.8 l of water is added to 2.5l of a 7.0 m koh solution, what is the molarity of the new solution
Answers: 1


Chemistry, 22.06.2019 17:30
Energy defines the different "states" of matter. in no more than 3 sentences, describe the amount of kinetic energy that each of the 3 states of matter possesses and relate that to the atom/molecular motion of each "state".
Answers: 2
You know the right answer?
What does Avogadro's law say about a gas atSTP? A; 1 L of any gas contains 22.4 moles at STP B; 1 L...
Questions



Social Studies, 07.07.2019 05:30


Social Studies, 07.07.2019 05:30

Biology, 07.07.2019 05:30

Social Studies, 07.07.2019 05:30

Biology, 07.07.2019 05:30

Social Studies, 07.07.2019 05:30




Mathematics, 07.07.2019 05:30

History, 07.07.2019 05:30

Mathematics, 07.07.2019 05:30


Mathematics, 07.07.2019 05:30



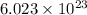 of particles.
of particles.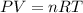
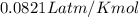
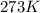 (at STP)
(at STP)