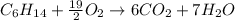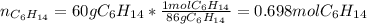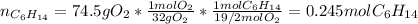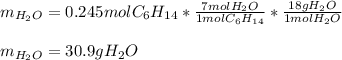Problem PageQuestion Liquid hexane CH3CH24CH3 will react with gaseous oxygen O2 to produce gaseous carbon dioxide CO2 and gaseous water H2O. Suppose 60. g of hexane is mixed with 74.5 g of oxygen. Calculate the maximum mass of water that could be produced by the chemical reaction. Round your answer to 3 significant digits.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 13:00
The molality of calcium chloride (cacl2) in an aqueous solution is 2.46 m. what is mole fraction of the solute?
Answers: 3


Chemistry, 22.06.2019 22:40
Covalent bonds generally form when the bonded elements have a difference in electronegativity less than 1.5. subtract the electronegativities for the following pairs of elements and predict whether they form a covalent bond. electronegativity difference of c and c: ionic covalent electronegativity difference of mg and cl: ionic covalent
Answers: 1

Chemistry, 23.06.2019 13:30
Explain the impact that changing the temperature has on a system in a state of dynamic equilibrium. what will happen when the temperature of an exothermic reaction mixture at equilibrium is increased?
Answers: 3
You know the right answer?
Problem PageQuestion Liquid hexane CH3CH24CH3 will react with gaseous oxygen O2 to produce gaseous c...
Questions

Mathematics, 29.04.2021 16:30

Mathematics, 29.04.2021 16:30

Mathematics, 29.04.2021 16:30

Chemistry, 29.04.2021 16:30


Mathematics, 29.04.2021 16:30

Chemistry, 29.04.2021 16:30

Mathematics, 29.04.2021 16:30

Physics, 29.04.2021 16:30






English, 29.04.2021 16:30

Computers and Technology, 29.04.2021 16:30


Biology, 29.04.2021 16:30

Mathematics, 29.04.2021 16:30