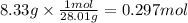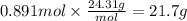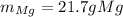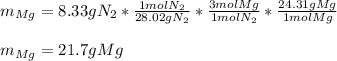Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 00:00
Which of the following statements is true? a. elements in the last period are radioactive. b. atomic weight is the same as atomic mass. c. elements in the same group have the same number of electron shells. d. atomic number equals the number of neutrons in the nucleus of an atom.
Answers: 1

Chemistry, 22.06.2019 14:00
How is the atomic number of a nucleus changed by alpha decay
Answers: 2

Chemistry, 22.06.2019 14:10
Precision can be defined as the o exact center of a data set. o reproducibility of a measured value. o correlation between two variables that are measured in a data set agreement between a measured value and an accepted value.
Answers: 2
You know the right answer?
Magnesium and nitrogen react in a combination reaction to produce magnesium nitride:
3 Mg + N2 → Mg...
Questions







Social Studies, 24.09.2019 00:00




Mathematics, 24.09.2019 00:00


Mathematics, 24.09.2019 00:00


Mathematics, 24.09.2019 00:00