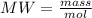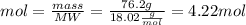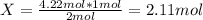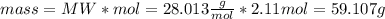Chemistry, 12.06.2020 01:57 alshaibanihassan10
The substances nitrogen monoxide and hydrogen gas react to form nitrogen gas and water. Unbalanced equation: NO (g) + H2 (g) N2 (g) + H2O (l) In one reaction, 76.2 g of H2O is produced. What amount (in mol) of H2 was consumed? What mass (in grams) of N2 is produced?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Which of the following two events occur to create a sea breeze? select all that apply. warm air rises on the ocean and moves toward the land to cool warm air rises on land and moves toward the ocean to cool cool air moves from the ocean to be warmed by the land cool air moves from the land to be warmed by the ocean
Answers: 3

Chemistry, 22.06.2019 05:30
Choose all the answers that apply. as ocean depth increases, temperature decreases temperature increases pressure increases pressure decreases salinity increases density increases
Answers: 2

Chemistry, 22.06.2019 20:30
The activation energy for the reaction no2(g)+co2(g)⟶no(g)+co(g) is ea = 300 kj/mol and the change in enthalpy for the reaction is δh = -100 kj/mol . what is the activation energy for the reverse reaction?
Answers: 3

Chemistry, 22.06.2019 23:00
Arectangle has a diagonal 20 inches long that forms angles of 60 and 30 with the sides. find the perimeter of the rectangle. for geometry
Answers: 3
You know the right answer?
The substances nitrogen monoxide and hydrogen gas react to form nitrogen gas and water. Unbalanced e...
Questions

SAT, 23.06.2019 11:00



Mathematics, 23.06.2019 11:00




Mathematics, 23.06.2019 11:00

History, 23.06.2019 11:00

Health, 23.06.2019 11:00

Mathematics, 23.06.2019 11:00

History, 23.06.2019 11:00

Geography, 23.06.2019 11:00

Mathematics, 23.06.2019 11:00

Mathematics, 23.06.2019 11:00

Mathematics, 23.06.2019 11:00

History, 23.06.2019 11:00



Mathematics, 23.06.2019 11:00