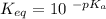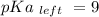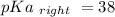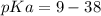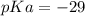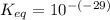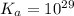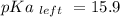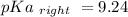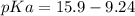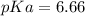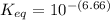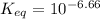Chemistry, 12.06.2020 04:57 katekayrodriguez10
Calculate Keq for these reactions and predict if the equilibrium will lie to the right or to the left as written. (You may enter your answer in scientific notation, e. g. 1.0*10^-9. Enter your answer to two significant figures.) Reaction 1: + + pKa = 9 pKa = 38 Keq = Equilibrium position = Reaction 2: + + pKa = 35 pKa = 25 Keq = Equilibrium position =

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:00
What three natural resources are found in the great lakes region
Answers: 2

Chemistry, 22.06.2019 05:00
Frictional forces acting on an object are often converted into energy, which causes the temperature of the object to rise slightly.
Answers: 2

Chemistry, 22.06.2019 11:40
Consider this equilibrium: n29) + o2(g) + 2no(c).nitrogen gas and oxygen gas react when placed in a closed container. as the reaction proceeds towards equilibrium, what happens to the rate of thereverse reaction?
Answers: 1

Chemistry, 22.06.2019 20:30
Draw a line graph showing the relationship between temperature in kelvin as a function of kinetic energy.
Answers: 3
You know the right answer?
Calculate Keq for these reactions and predict if the equilibrium will lie to the right or to the lef...
Questions

Mathematics, 21.06.2019 19:50

Mathematics, 21.06.2019 19:50





Biology, 21.06.2019 19:50



Biology, 21.06.2019 19:50

English, 21.06.2019 19:50

Mathematics, 21.06.2019 19:50






Geography, 21.06.2019 19:50


English, 21.06.2019 19:50

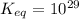
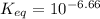
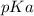 is mathematically evaluated as
is mathematically evaluated as 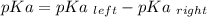
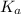 is mathematically evaluated as
is mathematically evaluated as