
Chemistry, 12.06.2020 15:57 carmencolon119
Given a K value of 0.43 for the following aqueous equilibrium, suppose sample Z is placed into water such that it’s original concentration is 0.033 M. Assume there was zero initial concentration of either A(aq) or B(aq). Once equilibrium has occurred, what will be the equilibrium concentration of Z? 2A(aq) + B(aq) <> 2Z (aq)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Zoe is investigating the composition of substance a, an unknown substance. using chemical processes, she analyzes substance a and determines it is composed of sodium, oxygen, and hydrogen atoms in a ratio of 1 : 1 : 1. what is substance a? a. a compound b. an element c. a heterogeneous mixture d. a homogeneous mixture
Answers: 1

Chemistry, 22.06.2019 09:40
Which diagram shows the correct way to represent an ionic compound of magnesium oxide?
Answers: 3

Chemistry, 22.06.2019 16:40
The diagram below shows the movement of particles. what does this piece of evidence best support? the collision theory the maxwell-boltzmann distribution the effect of pressure on reaction rates the effect of temperature on reaction rates
Answers: 3

Chemistry, 22.06.2019 22:30
What relationship exists between an enzyme and a catalyst?
Answers: 1
You know the right answer?
Given a K value of 0.43 for the following aqueous equilibrium, suppose sample Z is placed into water...
Questions

Mathematics, 21.11.2020 09:20


Chemistry, 21.11.2020 09:20


Mathematics, 21.11.2020 09:20




Mathematics, 21.11.2020 09:20


History, 21.11.2020 09:20

Chemistry, 21.11.2020 09:20

Mathematics, 21.11.2020 09:20


Mathematics, 21.11.2020 09:20

Mathematics, 21.11.2020 09:20

Mathematics, 21.11.2020 09:20



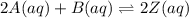
![K=\frac{[Z]^2}{[A]^2[B]}](/tpl/images/0684/0872/5d1cf.png)
![\frac{1}{K} =\frac{[A]^2[B]}{[Z]^2}=2.33](/tpl/images/0684/0872/c65f3.png)
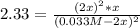
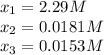
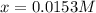 since the other solutions make the equilibrium concentration of Z negative which is not possible. In such a way, its concentration at equilibrium is:
since the other solutions make the equilibrium concentration of Z negative which is not possible. In such a way, its concentration at equilibrium is:![[Z]_{eq}=0.033M-2*0.0153M=0.0024M](/tpl/images/0684/0872/d1dc4.png)


