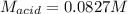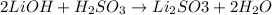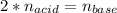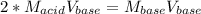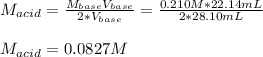Chemistry, 11.06.2020 20:57 DeGeneral770
A chemist uses a standard solution of 0.210 M lithium hydroxide (LiOH) to titrate 28.10 mL of sulfurous acid acid (H2SO3), she finds that it requires 22.14 mL of the base to reach the end-point of the titration. What is the molarity of the acid solution? What is the concentration of H2SO3? Your answer must be rounded to the correct number of significant figures. Be sure to specify a unit.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:10
Between 2014 and 2016, more than 25,000 children in flint, michigan, drank water that was contaminated with lead from lead pipes. during this time, the city claimed the water was safe to drink. which of these actions could the city have taken to ensure that the drinking water was free from lead?
Answers: 3

Chemistry, 22.06.2019 04:30
Using the periodic table, complete the table to describe each atom. type in your answers
Answers: 3

Chemistry, 22.06.2019 10:00
3. how much energy in joules is required to evaporate .0005 kg of liquid ammonia to vapor at the same temperature? 4. how much energy ( in megajoules ) is given up by .75 kg of water at 0c when it freezes to form ice at 0c? 5. explain how heat works between and at critical temperatures?
Answers: 2

Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 1
You know the right answer?
A chemist uses a standard solution of 0.210 M lithium hydroxide (LiOH) to titrate 28.10 mL of sulfur...
Questions




Health, 25.06.2019 03:10



History, 25.06.2019 03:10



English, 25.06.2019 03:10

Mathematics, 25.06.2019 03:10

Social Studies, 25.06.2019 03:10

Computers and Technology, 25.06.2019 03:10

Chemistry, 25.06.2019 03:10


Mathematics, 25.06.2019 03:10

History, 25.06.2019 03:10

Mathematics, 25.06.2019 03:10


Mathematics, 25.06.2019 03:10